
In the equation above there are no numbers in front of the terms, so each coefficient is assumed to be one (1). Similarily, if you have a mole of carrots, you have 6.023 x 10 23 carrots.

If you have a dozen carrots, you have twelve of them. A mole is similar to a term like a dozen. A mole simply represents Avogadro's number (6.023 x 10 23) of molecules. The MoleGiven the equation above, we can tell the number of moles of reactants and products. The graphic below works to capture most of the concepts described above: For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe 2O 3, a temperature of 1000 degrees C, and a pressure of 500 atmospheres for this reaction to occur. This information, such as a value for temperature, show what conditions need to be present for a reaction to occur. On some occasions, a variety of information will be written above or below the arrows. If no coefficient is shown, a one (1) is assumed. This amount can represent either the relative number of molecules, or the relative number of moles (described below). Finally, the (g) sign means that the compound is a gas.Ĭoefficients are used in all chemical equations to show the relative amounts of each substance present. The (aq) sign stands for aqueous in water and means the compound is dissolved in water. The (l) sign means the substance is a liquid. The (s) sign means that the compound is a solid. Often chemical equations are written showing the state that each substance is in. Since they undergo a chemical process, they are changed fundamentally. The equation shows that the reactants (AgNO 3 and NaCl) react through some process (->) to form the products (AgCl and NaNO 3). In this equation, AgNO 3 is mixed with NaCl. For example:ĪgNO 3(aq) + NaCl(aq) -> AgCl (s) + NaNO 3(aq) You will have a chance to review naming schemes, or nomenclature, in a later reading.Ī chemical equation is an expression of a chemical process. We call chlorine "chloride" in this case because of its connection to sodium. To represent a molecule of table salt, sodium chloride, we would use the notation "NaCl", where "Na" represents sodium and "Cl" represents chlorine. For example, the symbol "C"represents an atom of carbon, and "H" represents an atom of hydrogen. Successin chemistry depends upon developing a strong familiarity with these basic symbols. What is a chemical equation?In chemistry, we use symbols to represent the various chemicals. Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation. This reaction requires students to balance the equation.Chemical StoichiometryStoichiometry is the accounting, or math, behind chemistry. The balanced symbol equation is 6CO 2 + 6H 2O → C 6H 12O 6 + 6O 2. The word equation for photosynthesis is carbon dioxide + water → glucose + oxygen. Photosynthesis: Endothermic reaction that produces glucose and oxygen.This reaction requires students to balance the equation. The balanced symbol equation is CH 4 + 2O 2 → CO 2 + 2H 2O. Methane and Oxygen: Combustion that forms carbon dioxide and water.The balanced symbol equation is 2H 2 + O 2 → 2H 2O The word equation for this reaction is hydrogen + oxygen → water. Hydrogen and Oxygen: Reaction that forms water.The balanced symbol equation is Fe + S → FeS. The word equation for this reaction is iron + sulfur → iron sulfide. Iron and Sulfur: Exothermic reaction that forms iron sulfide.Reaction of sulfur trioxide and water to form sulfuric acid - SO 3 + H 2O → H 2SO 4.Reaction between sodium and water to produce sodium hydroxide and hydrogen - Na + H 2O → NaOH + H 2.If you want to avoid this, choose the following alternative reactions: The final two reactions listed in the activity require students to use the additional skill of balancing the symbol equation with coefficients. The chemical reaction models suggested below get more difficult as students progress through. To extend this activity, ask students to add a cell that explains what type of reaction it is (exothermic vs endothermic) and what happens during the process.

They should be sure to include the reactants, products, and the equation in their finished product.
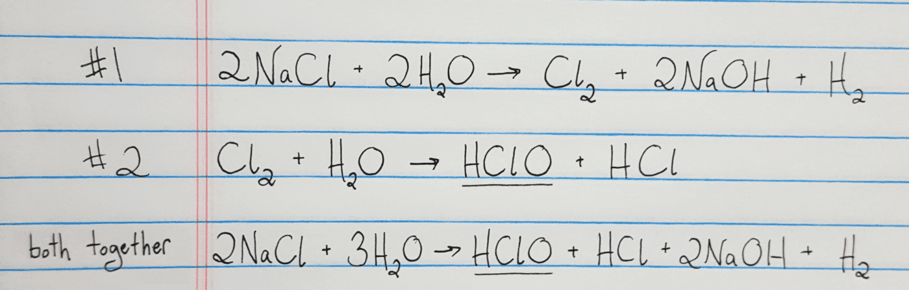
In this activity, students will create a grid that illustrates four chemical reactions. Creating models of chemical reactions is super important to help students understand how things change and how atoms balance.


 0 kommentar(er)
0 kommentar(er)
